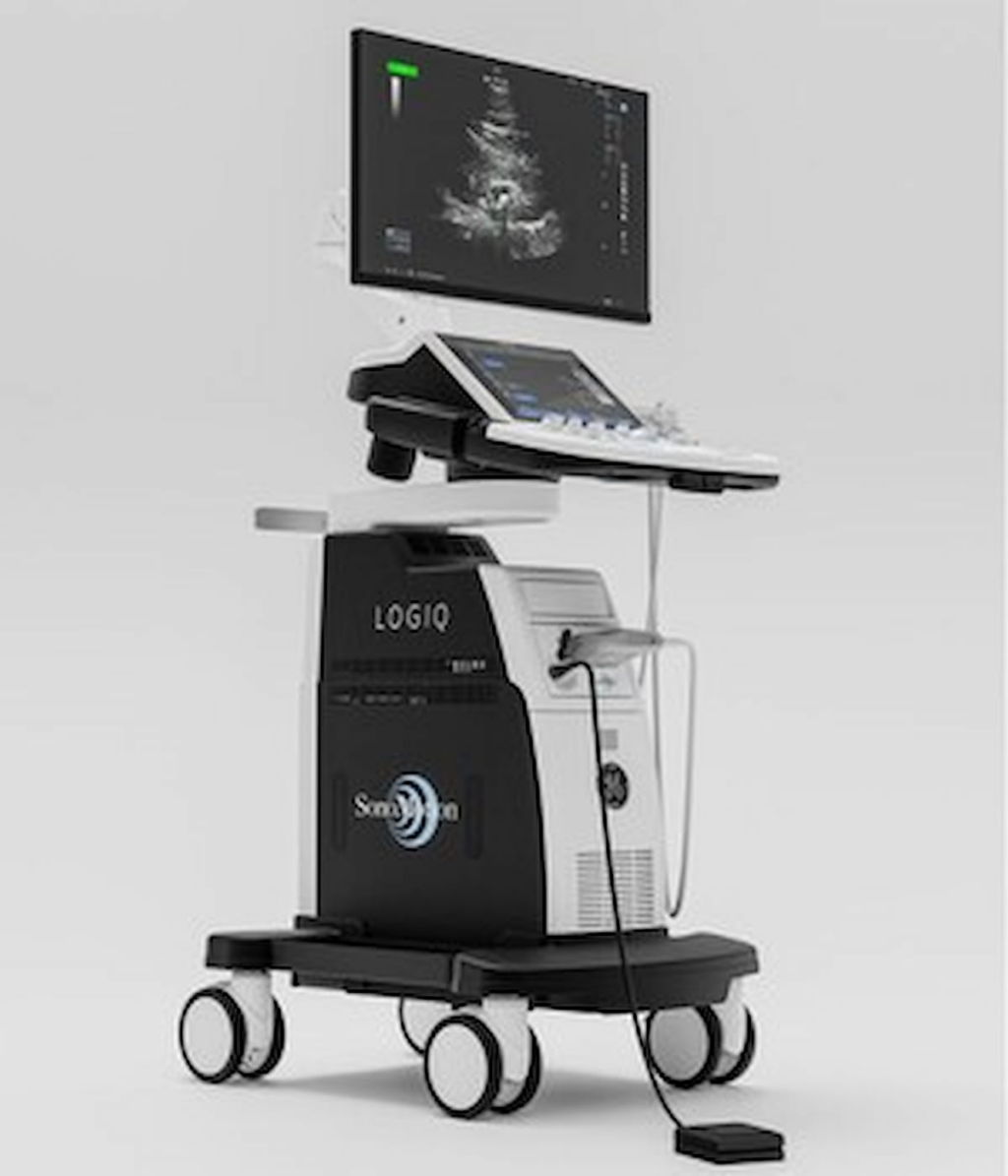
The approval of the Stone Clear device by the Food and Drug Administration (FDA) marks a significant advance in kidney stone treatment. This innovative tool employs ultrasound technology to assist in the passage of kidney stone fragments that remain following lithotripsy, a common procedure used to break down kidney stones.
Manufactured by SonoMotion, the Stone Clear device operates using ultrasound propulsion. This technology facilitates the movement of kidney stone fragments post-lithotripsy, making it easier for them to pass naturally through the urinary tract. A key advantage is that the procedure is non-invasive and does not require anesthesia, providing a significant improvement over traditional methods. Furthermore, the entire process can be efficiently completed in about 15 minutes, enhancing patient comfort and convenience.
Clinical trials have reinforced the efficacy and benefits of the Stone Clear device. A recent multicenter trial highlighted a striking 70% reduction in relapse risk for patients using ultrasound propulsion compared to those who were merely observed without intervention. This finding underscores the potential of Stone Clear to significantly mitigate the risk of complications associated with residual kidney stone fragments.
Dr. James E. Lingeman, a respected clinical professor of urology at Indiana University School of Medicine, emphasized the importance of such a non-invasive solution. According to Dr. Lingeman, patients with residual fragments post-lithotripsy face increased risks of health complications, emergency room visits, and often need additional procedures. Prior to innovations like Stone Clear, patients were largely left with passive observation as their only option. This device now offers a proactive, clinic-based solution that can be administered while patients are fully awake and conscious.
In light of these developments, the Stone Clear device represents a promising step forward in the management and treatment of kidney stones. With its ability to reduce the burden of residual stone fragments, it provides a valuable option for improving patient outcomes and potentially decreasing the overall healthcare burden associated with kidney stone management.