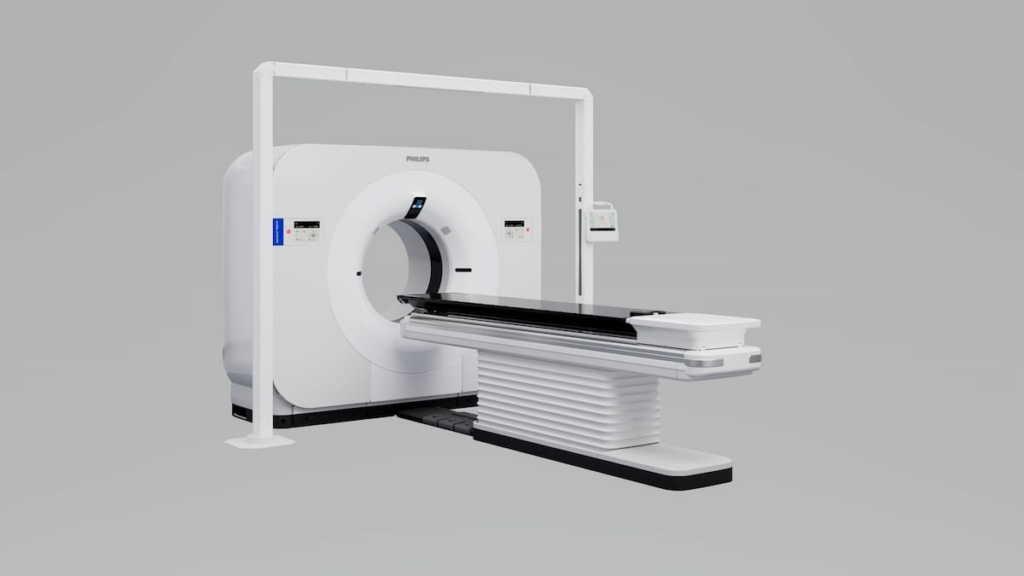
The Food and Drug Administration (FDA) has recently approved the Spectral CT 7500 RT system through a 510(k) clearance, providing clinicians with an innovative tool that combines the functions of 4D conventional computed tomography (CT) and spectral CT in a single scan. Designed specifically for radiation oncology, this system by Philips enhances clinicians’ ability to characterize tissues, thereby facilitating more precise radiotherapy targeting.
Philips, the creator of the Spectral CT 7500 RT, emphasizes that this newly cleared system is pivotal in improving targeted radiotherapy. By leveraging spectral CT technology, it allows for accurate mapping of tissue characteristics, which plays a critical role in the precision of radiation doses administered during treatment. Notably, the system’s ability to create automated stopping-power ratio (SPR) mappings and direct electron density readings significantly refines the dosing accuracy, an advancement that could potentially lead to better patient outcomes in radiation therapy.
A key feature distinguishing the Spectral CT 7500 RT is that it is the inaugural radiation therapy CT platform to integrate respiratory-gated spectral imaging. This integration offers enhanced mapping capabilities, such as SPR mapping and direct electron density, optimizing the calibration required for accurate radiotherapy dosing. These capabilities open new avenues for minimizing the uncertainties often encountered during treatment planning phases.
Dr. Zhong Su, who is the Director of Physics in the Department of Radiation Oncology at the University of Arkansas for Medical Sciences Medical Center in Little Rock, acknowledges the enhanced functionalities of the Spectral CT system over conventional CT methods. According to Dr. Su, this advanced technology provides clinicians with analytical data, such as electron density and effective atomic number results, which can be translated into the proton stopping-power ratio. The valuable data gleaned from this advanced imaging system reportedly reduces uncertainties, moving away from conventional calibration curves and therefore, diminishing uncertainty margins during the planning of treatments.
Philips has announced that the Spectral CT 7500 RT system will gain visibility at the upcoming Radiological Society of North America (RSNA) Annual Meeting, which will be held in Chicago between December 1-5, 2024. This event is anticipated to draw attention to this groundbreaking technology and its potential impact on the field of radiation oncology, showcasing its benefits to an audience of professionals eager for innovation that promises enhanced precision and safety in patient care.