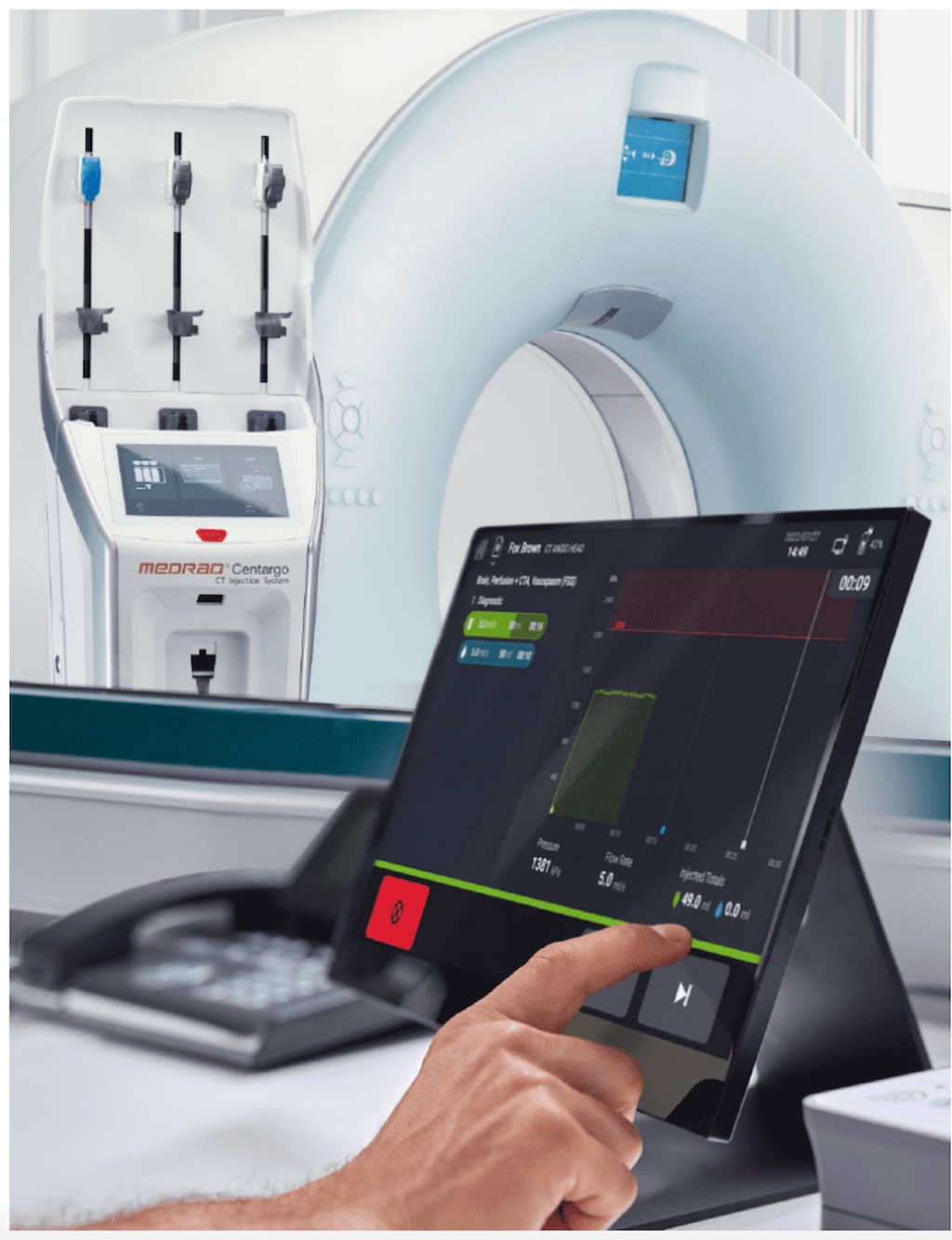
The Medrad Centargo CT Injection System, manufactured by Bayer, has recently received 510(k) clearance from the Food and Drug Administration (FDA). This new system is designed to streamline the process of administering contrast agents during computed tomography (CT) exams, allowing for efficient management of multiple patients.
One of the standout features of the Medrad Centargo CT Injection System is its pre-assembled Day Set and a user-friendly snap-in patient line, which automatically primes itself upon insertion. These design elements significantly reduce the time needed for contrast set-up to under two minutes for multiple patients, enhancing the overall workflow in busy radiology departments.
Bayer has emphasized the enhanced capabilities of the system when dealing with complex CT protocols. It is equipped with a Dual Flow feature that permits the simultaneous delivery of contrast media and saline. This is made possible through the system’s advanced piston-based technology, ensuring precision and efficiency during contrast administration.
The Medrad Centargo CT Injection System also integrates automated documentation software along with a barcode reader. This combination simplifies the recording and accessing of contrast and injection data, which in turn facilitates a more streamlined reporting process for medical professionals.
Sven Schmidt, Bayer’s head of Region Americas Radiology, highlighted the system’s benefits, noting the growing challenges faced by radiologists due to increasing workloads. He pointed out that the automation, integration, and mobility offered by the Medrad Centargo system provide radiology staff with the tools to operate more effectively and focus more on patient care.
Overall, Bayer’s Medrad Centargo CT Injection System appears to be a significant advancement in CT imaging, offering improved efficiency and functionality to support medical professionals in delivering quality patient care.