The U.S. Food and Drug Administration (FDA) has officially cleared icobrain.aria, a software powered by artificial intelligence, designed to enhance the detection of amyloid-related imaging abnormalities (ARIAs) on brain MRIs in Alzheimer’s disease patients. Icometrix, the innovative company behind this technology, emphasizes that icobrain.aria is the first AI tool tailored specifically to diagnose and monitor ARIAs in individuals undergoing treatment for Alzheimer’s.
ARIAs have recently come into sharper focus following the approval of new treatment options aimed at modifying the progression of Alzheimer’s disease. This context underlines the significance of the FDA greenlighting such specialized AI technology. The purpose of icobrain.aria is to provide healthcare professionals with an advanced tool to improve accuracy in tracking these abnormalities during treatment, ultimately supporting better clinical decisions and patient safety.
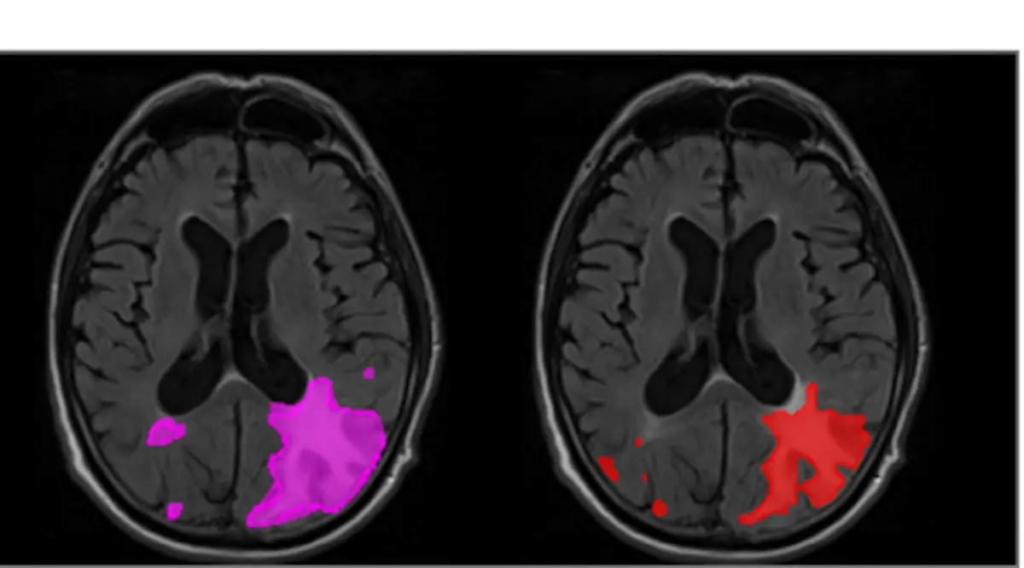
A retrospective study published in JAMA Network Open earlier this year has shed light on the capabilities of icobrain.aria, showcasing its increased sensitivity when detecting ARIAs. Specifically, the software demonstrated a 16% improvement in sensitivity for ARIA-E, which are edema-related abnormalities, and a 10% improvement for ARIA-H, or hemorrhage-related abnormalities. The study, which highlights images revealing severe ARIA-E manifesting as parenchymal edema, positions icobrain.aria as a markedly enhanced diagnostic tool compared to previous practices.
Dr. Stephen Salloway, director of neurology and head of the Memory and Aging Program at Butler Hospital in Providence, R.I., expressed optimism about the FDA’s clearance, stating the necessity for standardized tools that aid radiologists and clinicians in detecting and managing ARIAs effectively. With icobrain.aria now approved for broader clinical application, there is potential for more consistent and reliable management of these abnormalities, offering heightened safety for patients receiving treatment.
Considering the challenges in diagnosing and treating Alzheimer’s disease, incorporating AI technology such as icobrain.aria represents an essential step forward. As Alzheimer’s treatments advance, the capacity to monitor and understand associated imaging abnormalities becomes increasingly crucial. This advancement not only holds promise for optimizing safety but also for improving the overall quality of patient care. Thus, the integration of icobrain.aria into clinical practice marks a pivotal moment in the intersection of technology and healthcare, promising to enhance the capabilities of medical professionals dealing with complex neurological conditions like Alzheimer’s disease.