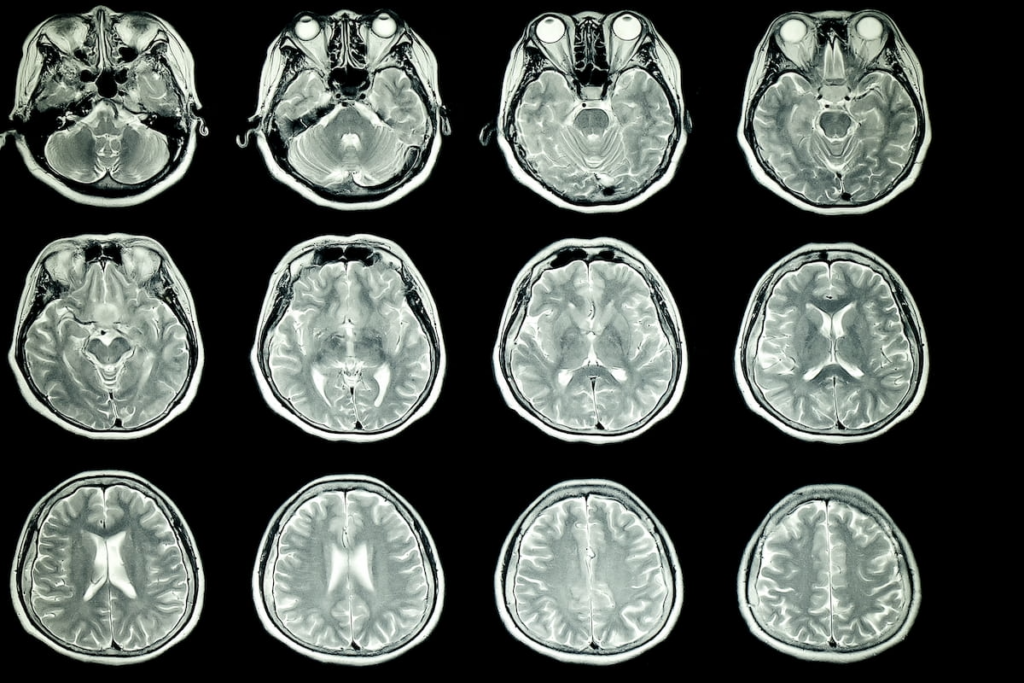
The U.S. Food and Drug Administration (FDA) has expanded its 510(k) clearance for the revolutionary artificial intelligence (AI) tool, Neurophet AQUA, designed to improve the assessment of multiple sclerosis (MS) utilizing magnetic resonance imaging (MRI). Neurophet AQUA, developed by Neurophet, originally gained FDA approval for evaluating brain atrophy through MRI scans. However, its capabilities have now been broadened to include advanced analysis concerning multiple sclerosis and white matter hyperintensities by leveraging T2-weighted fluid-attenuation inversion recovery (FLAIR) MRI technology.
Neurophet AQUA’s AI-powered analytics can quantify lesions and structural anomalies related to MS, offering a volumetric evaluation of brain regions, eliminating the need for 3D T1 imaging. This technological advancement facilitates the precise assessment of lesion count, volume, and progression, enhancing the quantification of MS-associated changes. Utilizing both 2D and 3D segmentation for T2-weighted FLAIR images across 1.5T and 3T MRI systems, Neurophet AQUA provides detailed volumetric analysis and improved image segmentation.
Jake Junkil Been, co-CEO of Neurophet, emphasized the central role of MRI in diagnosing and monitoring MS, highlighting that the McDonald criteria for MS diagnosis require MRI evidence of lesions disseminated both spatially and temporally. With Neurophet AQUA, practitioners receive advanced diagnostic capabilities that streamline the process, offering better efficiency and convenience, thereby proving indispensable at both diagnostic and prognostic stages of the disease. Such progress helps healthcare professionals deliver more accurate and timely assessments, ultimately benefiting patient care in managing this neurological disorder.